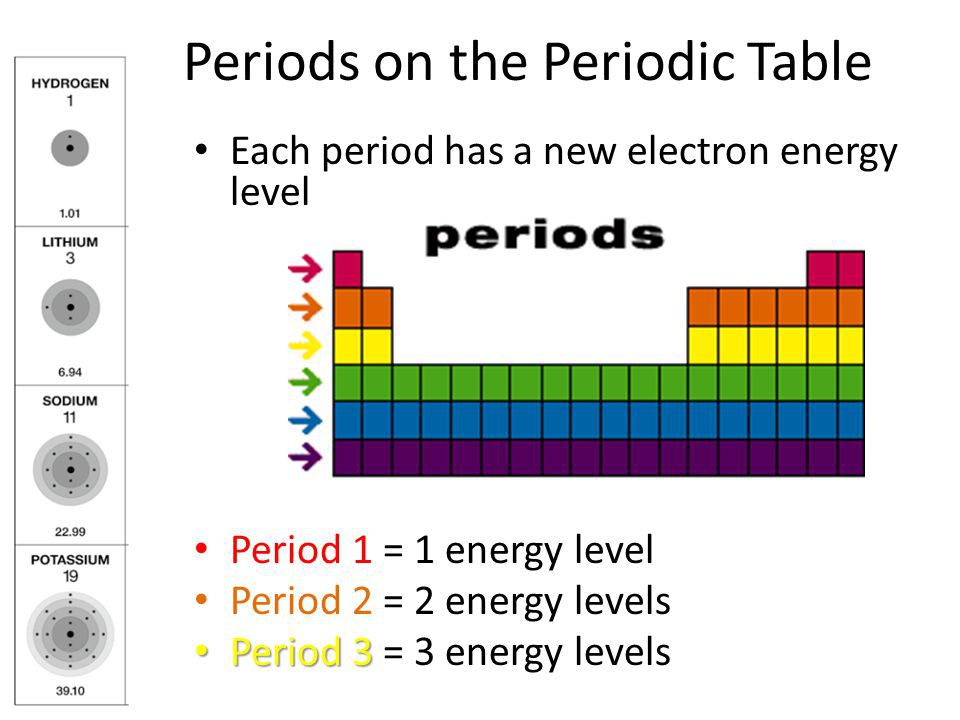
Energy Level In Periodic Table
Electron Configurations - Orbitals, Energy Levels and Ionisation Energy
Understanding the Basics of Electron Configurations
If you're studying chemistry, one of the most important things you need to learn is electron configurations. This refers to the arrangement of electrons in an atom or molecule. Understanding electron configurations is essential if you want to understand atomic structure, chemical bonding, and many other important topics in chemistry.
How to Calculate Electron Configurations
To calculate the electron configuration of an atom, you need to know the number of electrons it has and the order in which they fill different orbitals. The orbitals themselves are grouped into energy levels, each of which can accommodate a certain number of electrons. The first energy level, for example, can hold a maximum of two electrons, while the second energy level can hold up to eight.
How Electron Configurations Affect Chemical Properties
Why is it important to understand electron configurations? Because the number and arrangement of electrons in an atom or molecule will affect its chemical properties. For example, atoms in the same group (vertical column) of the periodic table have similar electron configurations and therefore similar chemical properties. Similarly, atoms in the same period (horizontal row) have different electron configurations and hence different chemical properties.
Why does atomic radii decrease going from the bottom left to the upper
Understanding Atomic Radii
One of the most important concepts in atomic structure is atomic radii. An atom's radius can be defined as the distance from its nucleus to the outermost shell of electrons. Atomic radii can vary depending on the atom's size and the number of electrons they have. In general, atomic radii increase as you move down a column in the periodic table and decrease as you move across a row.
How Atomic Radii Affect Chemical Properties
Why do atomic radii decrease as you move across a row in the periodic table? Because as the atomic number (number of protons) increases, so does the nuclear charge, or the attractive force that pulls electrons towards the nucleus. This makes the electrons more tightly bound to the nucleus, and therefore, reduces the atomic radii. Conversely, as you move down a column, the outermost electrons are further from the nucleus, making the atomic radii larger.
Properties of Atoms and the Periodic Table
Understanding the Periodic Table
The periodic table is a table that arranges all known chemical elements in order of increasing atomic number. The rows, or periods, in the periodic table indicate the number of energy levels an atom has. The columns, or groups, indicate the number of valence electrons the atoms in that group have. Understanding the arrangement of the elements in the periodic table is essential for understanding the properties of the elements and how they relate to each other.
How the Periodic Table Helps Predict Chemical Properties
The periodic table helps predict the chemical properties of the elements because elements in the same group have the same number of valence electrons, which determines their reactivity. For example, elements in group 1 (the alkali metals) all have one valence electron, making them highly reactive with other elements. Conversely, elements in group 18 (the noble gases) have a full outer shell of electrons, making them largely inert and unreactive.
Electron Configuration Overview & Examples
Using Electron Configurations to Predict Chemical Properties
As we discussed earlier, by understanding electron configurations, we can predict the chemical properties of the elements. For example, elements with a partially filled outermost shell of electrons tend to be more reactive. Conversely, elements with a full outermost shell of electrons tend to be inert.
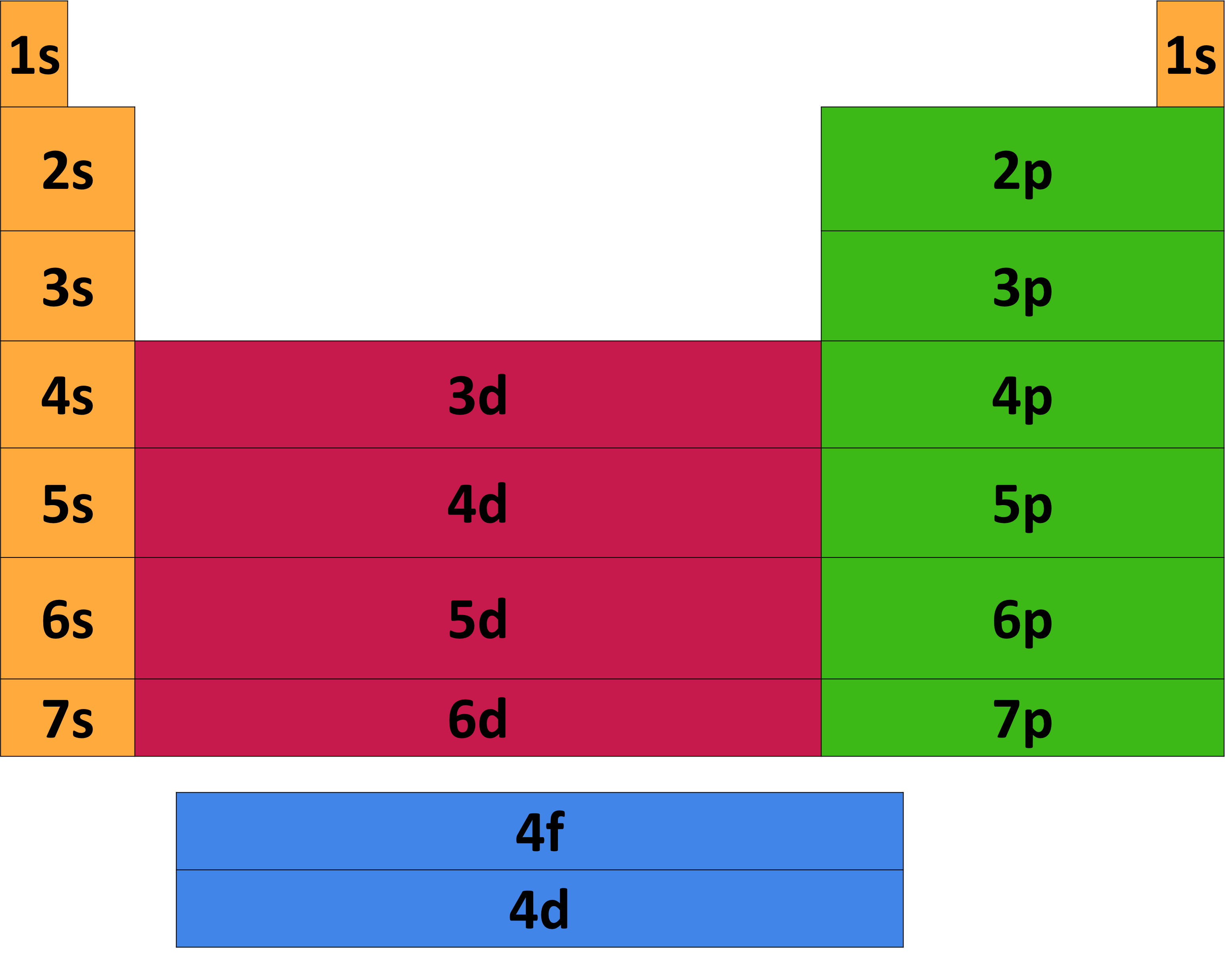
How to Use the Periodic Table to Determine Electron Configurations
The periodic table provides a systematic way of determining an element's electron configuration. By looking at the element's atomic number, we can determine how many electrons the element has. We can then use the periodic table to determine the order in which the electrons fill the various energy levels and subshells. The end result is a complete electron configuration for the element, which tells us a great deal about its chemical properties.
Periodic Trends in Ionization Energy
Understanding Ionization Energy
Ionization energy is the amount of energy required to remove an electron from an atom or molecule. It is an important concept in chemistry because it determines the reactivity of the atom or molecule. Elements with a low ionization energy are more likely to lose electrons and become positively charged ions, while elements with a high ionization energy are more likely to hold onto their electrons.

How to Use the Periodic Table to Predict Ionization Energy
The periodic table provides a way of predicting ionization energy because it follows a predictable pattern. Generally, ionization energy increases as you move across a row in the periodic table, because as we discussed earlier, the nuclear charge increases, making it more difficult to remove electrons. Conversely, ionization energy decreases as you move down a column in the periodic table, because the valence electrons are further from the nucleus, making them easier to remove.
By understanding these concepts, you can gain a deeper appreciation for the properties of the elements and the ways in which they interact with each other. Whether you're studying chemistry professionally or simply have an interest in the subject, it's well worth taking the time to understand electron configurations, atomic radii, the periodic table, and ionization energy. By doing so, you'll be well on your way to developing a deep understanding of the fundamental principles of chemistry.
Find more articles about Energy Level In Periodic Table