Columns On The Periodic Table -
Grasp the Periodic Table of Elements with Funny Mnemonics in Hindi
Hari ke hotey hai kuch naam, baki sab bhadey ka tamasha hai. That's how some of us remember elements and their positions in the Periodic Table. It's just a glimpse of how effective mnemonics are in remembering this fascinating, all-important scientific tool.
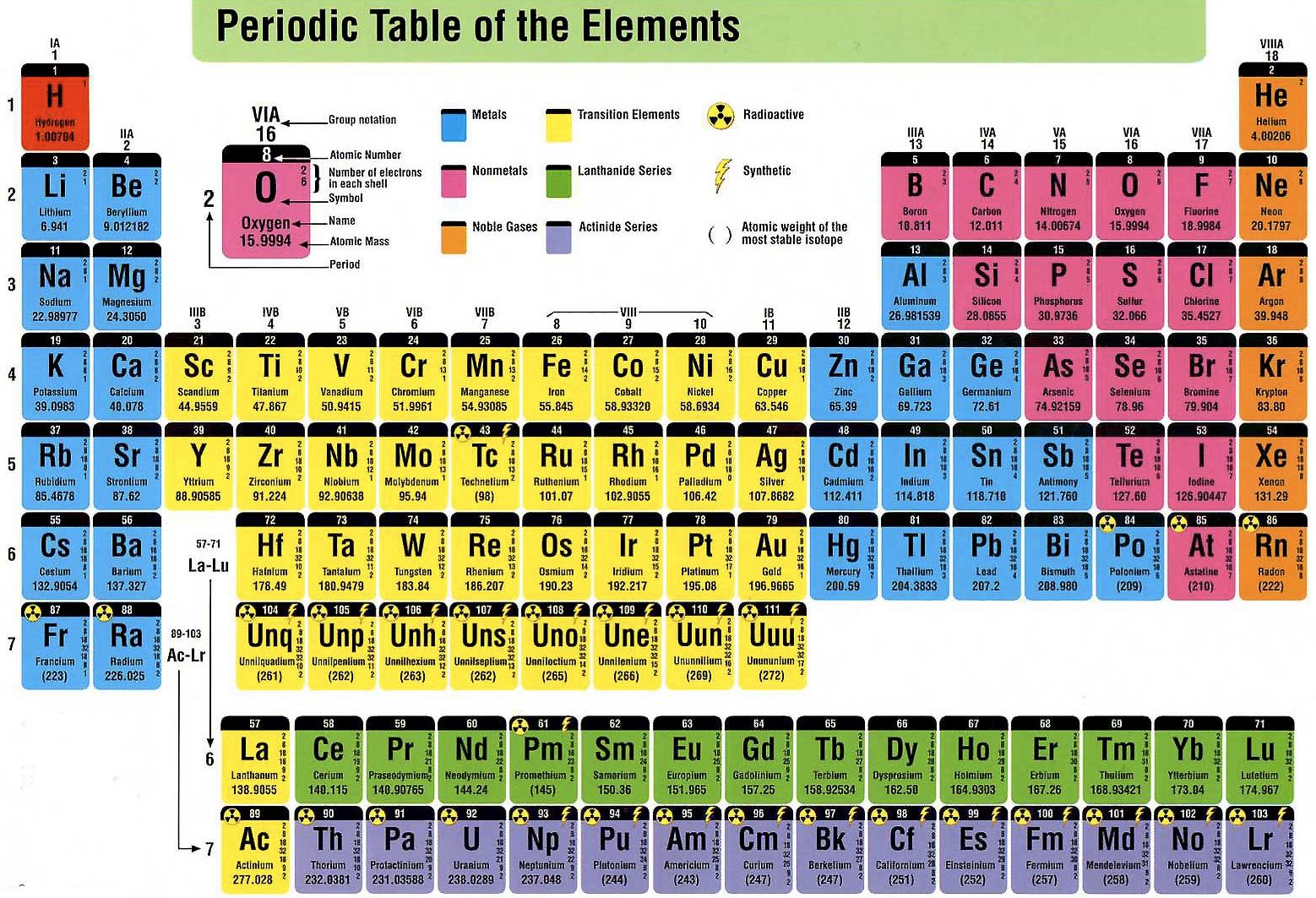
Here are some more:
- Hydrogen(H) - Hello, He Li (Lit. translated in Hindi), Be Kan! (Starts a conversation as such with the first few elements)
- Boron (B) - Baadaam (Almond), Jaamun (Jamun) - B-J (Both fruit names start with J)
- Carbon (C) - Cut Cut Carbon ke naam se, Saare satya ko bhar do ke baat se (Cut Cut is a famous tagline from a popular TV show)
Rhymes, acronyms, visual association, whatever works best for you. Remember, the periodic table relies heavily on memorization. But don't be intimidated. Mnemonics and other tricks will help you retain and make sense of the element's positioning on the table.
The Vertical Columns Of Elements In Periodic Table Are Called Brainly
Yes, the vertical column in the periodic table is called Group. But Brainly? That's a fun trick to remember. Brainly is a popular app for asking and answering homework questions. So, how does it come in handy with the periodic table? Here's a group-wise breakdown:

- Group 1 elements are called alkali metals. Think of them as the "brain" or the initiator of all chemical reactions.
- Group 2 - Alkaline earth metals - also play a critical role in chemical reactions, forming strong bases in water.
- Group 17 - Halogens - react with Group 1 and 2 metals to form salts.
- Group 18 - Noble gases - unreactive, stable atoms, making them the "brainy" of the group.
Periodic Table Vertical Columns | Review Home Decor
The periodic table has 18 vertical columns, each representing a group of elements with shared properties. Here's a brief overview of each group:

- Group 1 - alkali metals - highly reactive, soft, low density but highly corrosive in air or water.
- Group 2 - alkaline earth metals - reactive, with higher densities than Group 1 and less corrosive.
- Group 3-12 - transition metals - metals with varying properties, can conduct heat and electricity well, highly malleable and ductile.
- Group 13 - Boron group - non-metals with varying chemical and physical properties.
- Group 14 - Carbon group - have four valence electrons, which means they can share them to create a stable bond with other elements.
- Group 15 - Nitogen group - highly volatile and reactive non-metals. Major elements include nitrogen, phosphorus, and arsenic.
- Group 16 - Chalcogens group - highly reactive nonmetals that form various ionic and molecular compounds.
- Group 17 - Halogens - highly reactive nonmetals that form salty solutions when mixed with water.
- Group 18 - Noble gases - inert gases, mostly used in balloons, lightbulbs, and other applications where stable gases are critical.
Periodic Table Labeled Rows And Columns
The periodic table is a magnificent tool for learning and predicting the properties of atoms and molecules. It has nine horizontal rows or periods, each representing a new energy level or shell. The table also has 18 vertical columns or groups, representing elements with similar properties. Here's a labeled version:
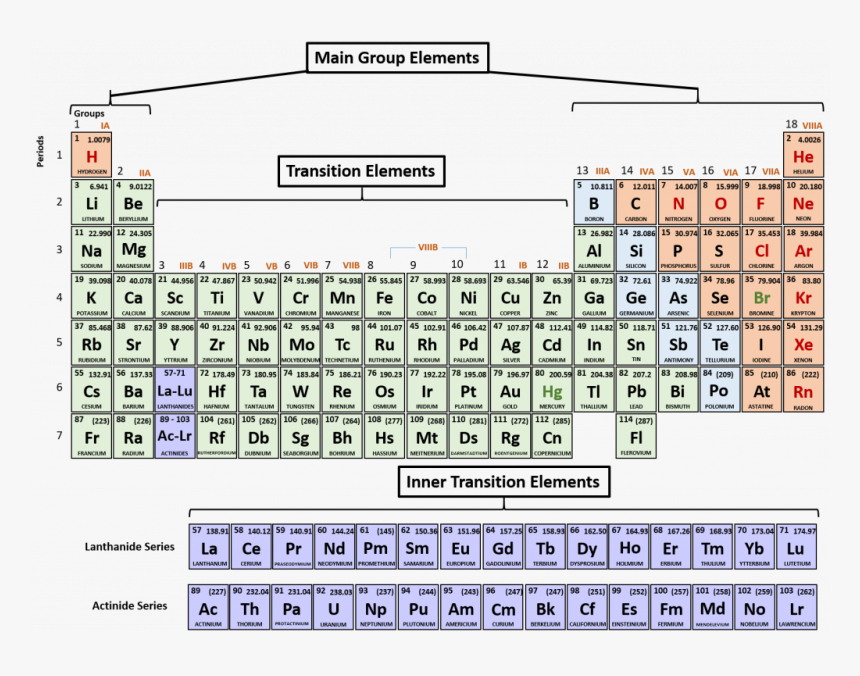
Here are some tips to keep in mind when using the periodic table:
- Elements in the same group have similar chemical and physical properties.
- Elements in the same row have the same number of electron shells.
- The atomic number represents the number of protons in the nucleus of an element, while the mass number represents the total number of protons and neutrons.
- The left side of the table has the most metal elements that give away electrons while the right side contains non-metals that attract electrons.
With these tips, you should be able to tackle any periodic table problem with ease. Remember, it's a tool meant to simplify the world of chemistry, not confuse it.
Find more articles about Columns On The Periodic Table