Trend Of Ionization Energy In Periodic Table - Have you ever wondered how scientists determine the tendency of an element to gain or lose an electron? If you have, then it’s time to learn about the trend of ionization energy in the periodic table. Throughout this post, we’ll be exploring different images that help to explain the trend of ionization energy in the periodic table. So, buckle up and let’s dive into the world of ionization energy!
Ionization: Ionization Trend
Understanding the Trend of Ionization Energy in the Periodic Table
The trend of ionization energy in the periodic table is determined by looking at how much energy is required to remove an electron from an atom. In general, the ionization energy of an element increases from left to right across a period, and decreases from top to bottom down a group. This means that elements on the left side of the periodic table have lower ionization energy compared to elements on the right side of the table. Similarly, elements at the top of the table have lower ionization energy compared to elements at the bottom of the table.
Ionization Energy: the amount of energy required to remove an electron
How to Measure Ionization Energy
Ionization energy is usually measured in units of electron volts (eV) or kilojoules per mole (kJ/mol). One electron volt is equivalent to the amount of energy required to move an electron through a potential difference of 1 volt. The higher the ionization energy of an atom, the more difficult it is to remove an electron from that atom. As a result, atoms with high ionization energy tend to have stable electron configurations and are therefore less reactive than atoms with low ionization energy.

All Periodic Trends in Periodic Table (Explained with Image)
Exploring All Periodic Trends
Aside from the trend of ionization energy, there are several other periodic trends that scientists use to understand the properties of elements. One of the most important of these trends is atomic radius, which refers to the distance between the nucleus of an atom and its outermost electrons.
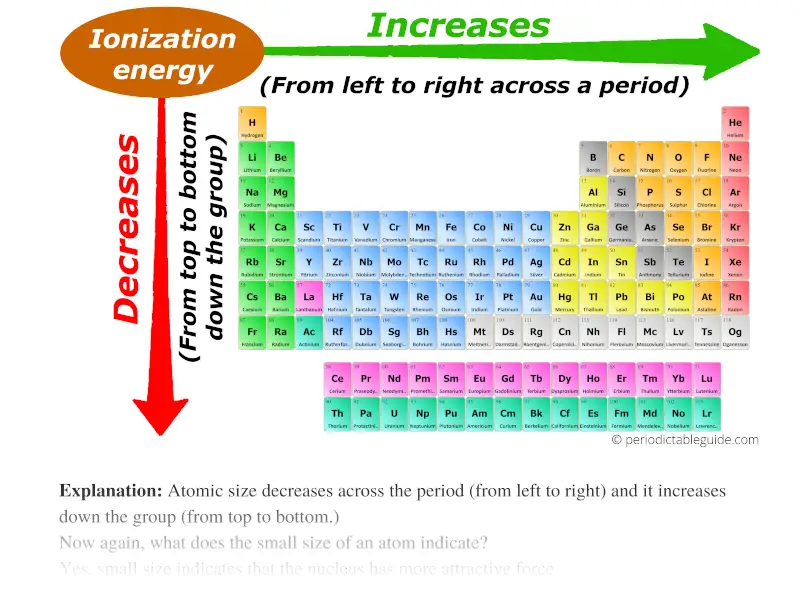
Ionization Enthalpy - NEET Lab
Understanding the Importance of Ionization Enthalpy
Ionization enthalpy is a measure of the amount of energy required to remove an electron from an atom in the gaseous state. It is also known as ionization energy or ionization potential. This property is of great importance in the study of chemistry, as it reflects the stability of an atom and its tendency to form chemical bonds.
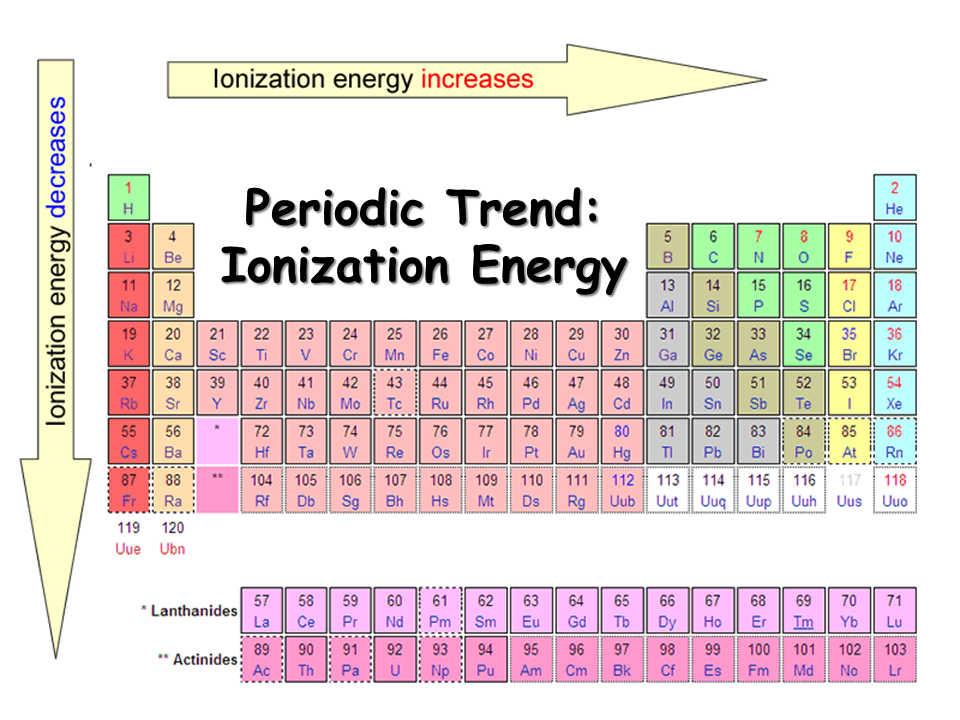
The Parts of the Periodic Table
Understanding the Structure of the Periodic Table
The periodic table is organized in a specific way to make it easier for scientists to understand the properties of elements. At the top of the table, there are two rows of elements that are separate from the other elements. These are called the lanthanides and actinides or the f-block elements. In the main body of the table, there are eight horizontal rows called periods and eight vertical columns called groups or families.

Tips and Ideas for Exploring the Trend of Ionization Energy
How to Get the Best Understanding
If you’re interested in learning more about the trend of ionization energy, there are a few tips and ideas that you can use to get the best understanding. First, make sure you have a good understanding of the basics of chemistry, including the properties of atoms and the periodic table. You can use the images in this post to help you visualize the trend of ionization energy and other periodic trends. Additionally, you can explore online resources, such as interactive periodic tables and educational videos, to deepen your understanding of this topic.
How to Use the Trend of Ionization Energy
Applying the Trend of Ionization Energy
The trend of ionization energy is useful in predicting the chemical properties of elements. For example, elements with low ionization energy tend to form cations, while elements with high ionization energy tend to form anions. Additionally, the trend of ionization energy can be used to predict the reactivity of elements. Elements with high ionization energy tend to be less reactive, while elements with low ionization energy tend to be more reactive.
In Conclusion
The trend of ionization energy in the periodic table is an important concept in the study of chemistry. Understanding this trend can provide insights into the chemical properties of elements and how they interact with one another. By exploring the images in this post and utilizing the tips and ideas provided, you can deepen your understanding of this topic and apply it to your studies or work as a scientist.
View more articles about Trend Of Ionization Energy In Periodic Table