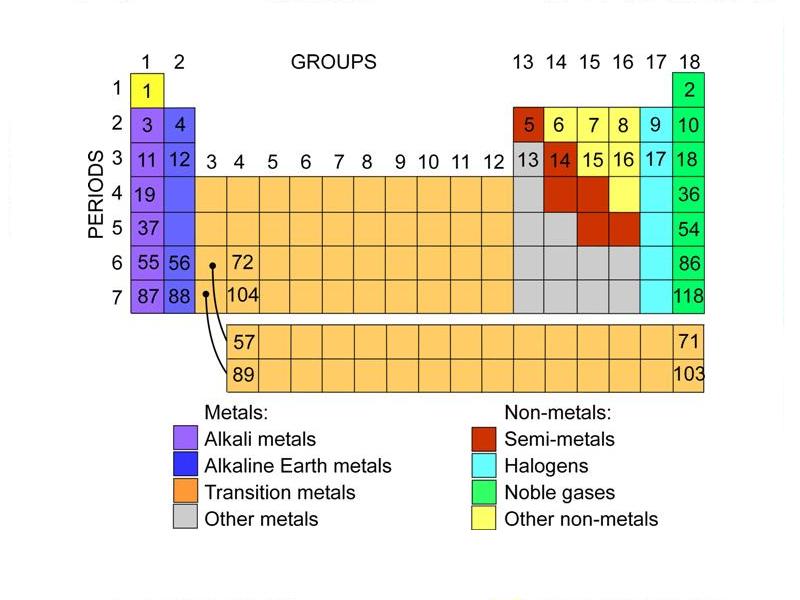
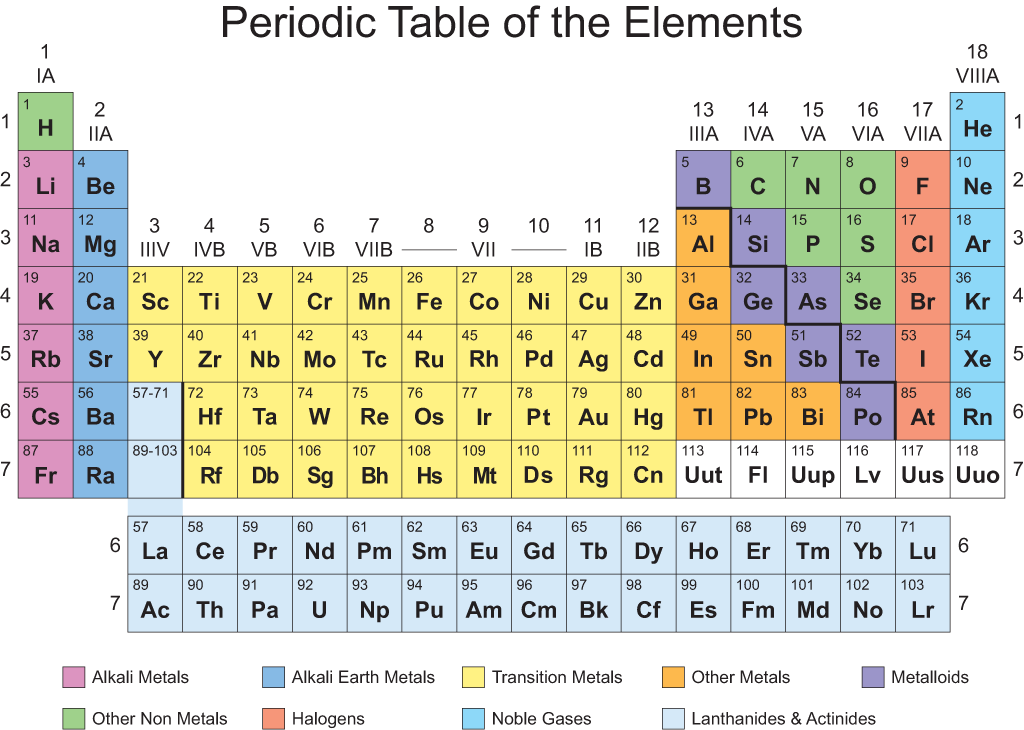
Groups On Periodic Table Of Elements - The periodic table of elements is a comprehensive representation of the chemical elements that exist. It lists all the elements that make up the universe in an organized manner based on their atomic structure, chemical properties, and other physical characteristics. The period table consists of 118 elements grouped into rows and columns depending on their electron configurations. While there are several ways to categorize the elements, one of the most common is based on their groupings. In this article, we'll explore the different groups on the periodic table of elements, their unique properties, and how they help us understand the behavior of the elements.
Group 1: Alkali Metals
Description:
The alkali metals are a group of elements that are very reactive and have only one electron in their outermost shell. They are the first column on the periodic table and include Lithium, Sodium, Potassium, Rubidium, Cesium, and Francium. These elements are soft, low-density metals that have a melting and boiling point lower than that of most other metals. They are never found in their pure form in nature and must be separated from their compounds.

Tips:
- Alkali metals react vigorously with water and can catch fire.
- They are often used in batteries, for treating depression, and in nuclear reactors.
- They have low electronegativity and ionization energy and are good conductors of heat and electricity.
Ideas:
- Make potassium soup by putting a small piece of potassium metal in water and sucking the vapors through a straw.
- Create a bomb with potassium by mixing it with water and throwing it at something. It can create an explosion with a flame temperature of over 900 degree Celsius.
How to:
If you want to store alkali metals for long periods, you can put them under oil or kerosene to prevent them from reacting with air or water.
Group 2: Alkaline Earth Metals
Description:
The second column on the periodic table contains the alkaline earth metals, which are less reactive than the alkali metals but still quite reactive. This group includes Beryllium, Magnesium, Calcium, Strontium, Barium, and Radium. These metals have two electrons in their outermost shell, which they readily lose to form positive ions. Like alkali metals, they are good conductors of heat and electricity.
Tips:
- Magnesium is used in pyrotechnics, while calcium is used in toothpaste.
- Alkaline earth metals are found in a wide range of minerals and are important for humans.
- Barium and Radium are naturally radioactive and can be very dangerous when ingested.
Ideas:
- Create a magnesium fire by mixing magnesium powder and methyl alcohol in a test tube and igniting it.
- Make a crystal radio with barium titanate by putting a small piece of the element onto a crystal diode.
How to:
Alkaline earth metals react slowly with water but quickly with acids. If you want to prevent this reaction, you can store them under a layer of mineral oil or in a vacuum.
Group 3-12: Transition Metals
Description:
The large block of elements in the middle of the periodic table is known as the transition metals. The elements in this group have complex electron configurations and are characterized by their ability to form multiple oxidation states. They are hard and dense metals with high melting and boiling points. This group includes Iron, Copper, Silver, Gold, Zinc, and Titanium, among others.
/the-periodic-table--digital-illustration--73016803-598b218ec41244001024af78.jpg)
Tips:
- Transition metals are used in jewelry making, construction, and electronics.
- Iron is the most common element on the planet and is used to make steel.
- Copper is a great conductor of electricity and is used in wiring as well as for making coins and sculptures.
Ideas:
- Create an alloy by mixing two or more transition metals together.
- Use zinc oxide in sunscreen to protect your skin from harmful UV rays.
How to:
Transition metals are often found in ores and must be extracted by various processes, including smelting and roasting. Once extracted, they can be purified using electrolysis or other methods.
Group 13-17: Other Non-Metals
Description:
These elements are located to the right of the transition metals and include elements like Carbon, Nitrogen, Oxygen, Fluorine, and Chlorine. They are called the other non-metals because they have properties different from the metals. They are generally poor conductors, have low melting and boiling points, and are highly reactive.
Tips:
- Oxygen is essential for life on Earth and is used in many industrial processes.
- Carbon is used in many applications, including pencil lead, diamond, and graphite.
- Fluorine is highly reactive and is used in toothpaste to prevent dental cavities.
Ideas:
- Create a carbon dioxide fire extinguisher by mixing dry ice and water in a plastic bottle.
- Make nitrogen ice cream by mixing liquid nitrogen with cream, sugar, and flavorings.
How to:
Other non-metals can be found in nature as pure elements, but they are often found in compounds. To extract them, different methods like electrolysis, reduction, and fractional distillation are used.
Group 18: Noble Gases
Description:
The last column of the periodic table contains the Noble Gases. These elements are inert and do not react easily with other elements. They have full electron shells and are located to the right of the transition metals. This group includes Helium, Neon, Argon, Krypton, Xenon, and Radon.

Tips:
- Noble gases are used in lighting, as they produce bright colors when an electric current passes through them.
- Helium is used in balloons as it is lighter than air.
- Krypton and Xenon are used in flashlights and headlights.
Ideas:
- Create a Noble gas light bulb by filling a glass globe with a Noble gas and passing an electric current through it.
- Use Helium to make your voice sound high pitched.
How to:
Noble gases are generally found in small quantities in the atmosphere and must be extracted by liquefaction or fractional distillation.
The periodic table of elements is an invaluable tool for scientists, chemists, and students. It allows us to understand the properties and behavior of the elements and is constantly being updated with new discoveries. By knowing the different groups on the periodic table of elements and their unique properties, we can learn more about the world around us and how it functions.
View more articles about Groups On Periodic Table Of Elements