What Are The Rows On The Periodic Table Called - If you're taking a chemistry class or just trying to expand your knowledge on the subject, you might have come across the term "periods" when referring to the rows in the periodic table. But have you ever wondered why they're called that? In this post, we'll take a closer look at the rows in the periodic table, their properties, and what makes them unique.
What Are The Rows in the Periodic Table?
The periodic table is a table that displays all of the known chemical elements in an organized fashion. The elements are arranged in order of their atomic number, which is the number of protons in the nucleus of an atom of that element. But not only are they arranged by atomic number, they're also arranged in rows, or periods.
Why Are They Called "Periods"?
The reason they're called "periods" is that the elements within a row share similar electronic shell configurations. That means that as you move across the row (from left to right), the number of electrons in the outermost shell (valence electrons) increases by one. This repeats itself every period, or full complete row, which is why it's called a "period".
Periods vs. Groups
It's important to note that periods are different from the groups, which are the vertical columns of the periodic table. The elements within a group share similar chemical properties and valence electron configurations, rather than electronic shell configurations.
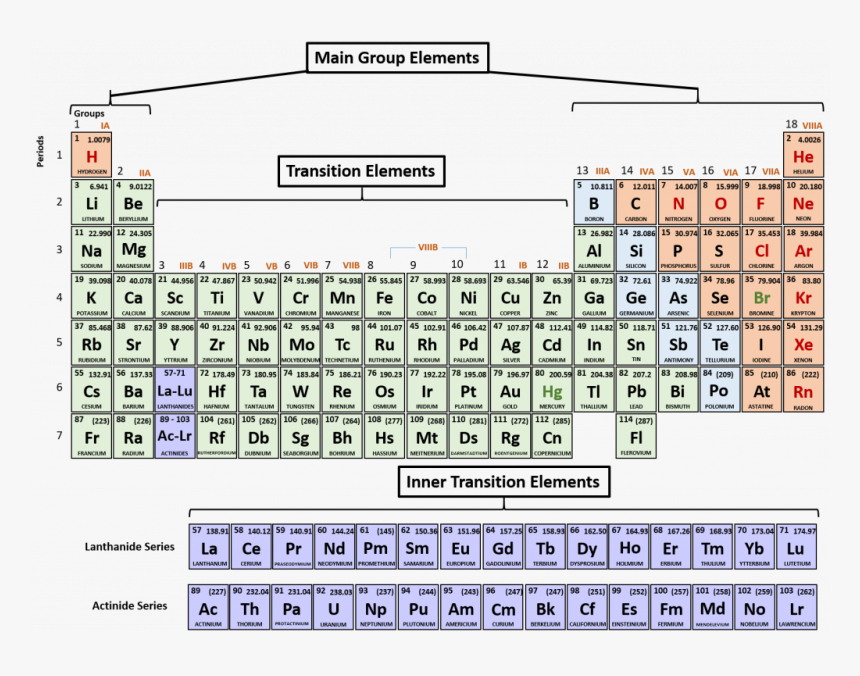
What Are Some Properties of Periods?
As mentioned earlier, elements within a row share similar electronic shell configurations. But what does that mean for their properties?
- The atomic radius of the elements decrease from left to right across a row
- The electronegativity of the elements increases from left to right across a row
- The ionization energy of the elements increases from left to right across a row
How to Use the Periodic Table
So now that you know what the rows, or periods, are in the periodic table, how can you use it to your advantage?
Identifying Elements
If you know the atomic number of an element, you can easily find it on the periodic table by looking for the corresponding row (period) and column (group).
Predicting Properties
As mentioned earlier, properties of an element can be predicted based on its location on the periodic table. For example, if you know an element is in the fourth row (period) and in the seventh column (group), you can predict that it will have a high ionization energy and electronegativity. This information can be useful when trying to identify unknown substances, among other things.
Understanding Chemical Reactions
The periodic table can also be used to understand chemical reactions. For example, if you know the chemical properties of one element and the chemical properties of another element that it reacts with, you can determine what products will be formed. This can be especially useful in organic chemistry, where a variety of complex reactions can occur.
In conclusion, the rows in the periodic table, or periods, are much more than just a line on the table. They represent important electronic shell configurations that are an essential part of understanding chemistry as a whole. Understanding these properties can help us predict the behavior of elements and compounds, and ultimately lead to a better understanding of the world around us.
View more articles about What Are The Rows On The Periodic Table Called